Ovarian cancer accounts for more deaths than any other cancer of the female reproductive system. This illness often begins with little or no symptoms, meaning patients and their doctors discover it when it’s too late for preventative treatment. Conducting successful ovarian cancer clinical trials can help us uncover new treatments and save lives — but we can’t do that without healthcare analytics.
According to the American Cancer Society, ovarian cancer ranks fifth in cancer deaths among women — a woman’s risk of getting ovarian cancer during her lifetime is about 1 in 78, and her lifetime chance of dying from ovarian cancer is about 1 in 108.
PurpleLab™ Cohort Report – Ovarian Cancer Diagnosis by Age and Gender
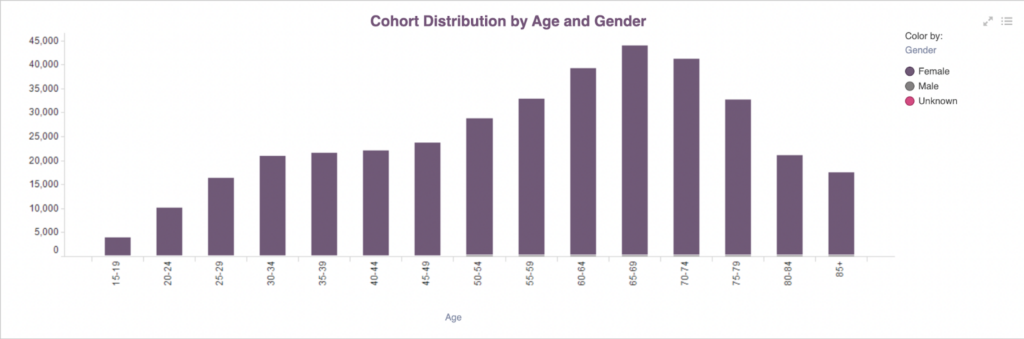
Ovarian cancer mainly develops in women ages 65-69 and women ages 75-79 are also at a high risk. However, all women, regardless of age, should go to annual doctor appointments and pay attention to their bodies. Some early signs of ovarian cancer include pelvic or abdominal pain or cramping, lack of appetite, indigestion, frequent urination and unexplained exhaustion. However, it’s common for symptoms to be mild, meaning diagnosis often comes when the illness is already at stage three or four.
It goes without saying that finding a cure is paramount. But because this discovery could be years away, we must also focus on creating a preventative treatment that works in the late stages of the disease. This requires conducting successful clinical trials and finding smarter, faster ways to parse, harmonize and navigate the data.
But to understand how and where healthcare analytics can help, let us first dive into the needs and challenges of ovarian cancer clinical trials.
What Is Needed for Successful Ovarian Cancer Clinical Trials?
Any successful clinical trial depends on the combination of three factors — eligible patients, their treating providers and accessible sites. For ovarian cancer clinical trials, this becomes even more complex, partially due to the increasingly personalized approach to ovarian cancer treatments.
The Right Patients
The right patients for a clinical trial need to meet specific criteria. Finding these patients requires an in-depth understanding of certain patient dimensions, which are listed below.
- Genetics — There are certain genetic variants that are associated with increased risk of ovarian cancer and are responsible for inherited susceptibility syndromes.
- Tumors & Immune Response — Tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs) in cancer vaccines are used to activate a patient’s immune system. These vaccines can activate cellular immunity and prevent tumor growth.
- Diagnosis Timing & Stage — Both the timing of diagnosis and the stage of cancer in patients can help us understand contributing factors, such as patient location and life habits.
- Comorbidities — Some women who have ovarian cancer have other medical conditions at the same time, which can impact their treatment. For example, this could be surgery, chemotherapy or other interventions. Also, the interactions of various treatments for comorbidities can lead to adverse side effects — this is known as risk adjustment factor (RAF).
- Age & Race — Women of certain ages and ethnicities are inherently more prone to developing ovarian cancer than others.
The Right Providers
Finding the right providers for a clinical trial can be just as difficult as finding the right patients. Significant ovarian cancer experience is required for a provider in this clinical trial scenario, but it’s also important to look at:
- Volume of ovarian cancer diagnoses at the provider’s practice
- Social determinants of health (SDOH) characteristics of the patients at that practice
- Practice locations
There will also need to be enough nurses and lab technicians to conduct a clinical trial successfully.
The Right Facilities
Finding the right facility goes hand-in-hand with both the right patients and providers. The facility used for a clinical trial needs to be at a convenient location for everyone involved, as the trial could last for weeks or months. If a chosen location is not convenient, patients and providers could end up backing out due to travel time.
What Are The Challenges With Ovarian Cancer Clinical Trials?
Clinical trials, regardless of their focus, are challenging. And when it comes to ovarian cancer clinical trials, certain nuances raise the bar even higher.
Challenge #1 — Very Expensive
Ovarian cancer clinical trials are highly expensive and unfortunately, underfunded. Data shows that the average cost to treat an ovarian cancer patient during a clinical trial is $13,802. This makes clinical researchers less likely to focus on ovarian cancer and further enforces the rise of ovarian cancer across the population. The less money we have to conduct these trials, the further away we will remain from a cure.
Challenge #2 — Patient Recruitment
Ovarian cancer is an aggressive disease, and the majority of suffering patients may not be interested in participating. The clinical trial could simply be too challenging for them, or they might be too sick to physically go to the trial location.
Additionally, when a patient does want to participate in a clinical trial, she has to meet the criteria mentioned above. Some clinical trials end up turning patients away because there are problematic comorbidities, the stage of cancer is too early or late, or a patient’s age doesn’t work with the trial group. The complex process makes patient recruitment as difficult as possible, especially when it comes to this particular cancer.
Challenge #3 — The Disease is Hard to Detect
Ovarian cancer is hard to detect – and it’s usually very advanced when it’s found. The drugs that are being developed for this disease are geared towards late-stage cancer that hasn’t responded to chemotherapy, and are typically for extending life as long as possible. With detection being so difficult, it’s nearly impossible to find patients with the right dimensions that are also in the preferred stages of diagnoses.
Using Healthcare Analytics to Improve Clinical Trial Success
Successful clinical trials are not possible without clean, conformed, reliable patient data. Software platforms that help synthesize and harmonize this data will be critical in helping ovarian cancer clinical trial sponsors and managers overcome the challenges above. Here’s how.
Harmonizing Data
Healthcare data is messy and often presents several issues including high volume and cost, a time-consuming ‘cleaning’ process, and a variety of coding requirements. If that weren’t enough, it must also comply with HIPAA. An effective healthcare analytics platform will offer tools for harmonizing data at the most clinical level. In other words, it translates data into the same medical language for all users, eliminating the heavy coding lift. Data harmonization helps research groups find patient data quickly and easily, streamlining the clinical trial patient recruitment process and connecting patients with the right providers and facilities.
SDOH
SDOH characteristics are vital in clinical trials. Economic stability, access to healthcare, overall environment and education access and quality are just a few of the conditions that matter most to researchers. A good platform should help you organize data accordingly, for every single patient.
HCP/HCO Targeting
A healthcare analytics platform shouldn’t just be able to find eligible patients. It should also be able to find eligible providers and other healthcare personnel (HCP). If your clinical trial is targeting patients in a certain region who are at a particular stage of ovarian cancer, your platform should tell you what providers are in that location and if they are qualified to participate in your clinical trial. Ensuring that a provider’s specialty aligns with all clinical trial criteria, and its focus treatment, saves time and money by avoiding outreach to ineligible providers.
Decentralized Clinical Trials
Healthcare analytics can also streamline Decentralized Clinical Trials (DCT), which offer alternatives to the face to face encounters in a standard trial. This clinical trial model allows patients enrolled in the trial to provide information in a remote manner, allowing people who live further from an academic research center to participate in the clinical trial. Utilizing this clinical trial model can significantly impact the recruitment of underrepresented groups in traditional trials, as they aim to support inclusiveness and convenience for everyone.
Backed by Clinical Experts
Not all healthcare data platforms are created equal. Many startups, for example, are created and run by professionals outside the clinical field. The best platforms are backed by actual clinical experts who understand clinical trial nuances and can support tricky queries and questions.
Conclusion
The more a pharmaceutical company knows about the patients, the better it will be at aligning those patients with clinical trial treatments. Streamlining clinical trials starts with finding better ways to connect the dots between patients and the treatments they need. And that means finding a healthcare analytics platform that makes it easy.
Conducting successful ovarian cancer clinical trials is a complex process that involves a significant amount of time, research and funding. PurpleLab™ powered by HealthNexus™ can help you access and leverage the data you need. Contact us today to learn more.